Machine Learning Signal Detection Performance Calculator
How Machine Learning Outperforms Traditional Methods
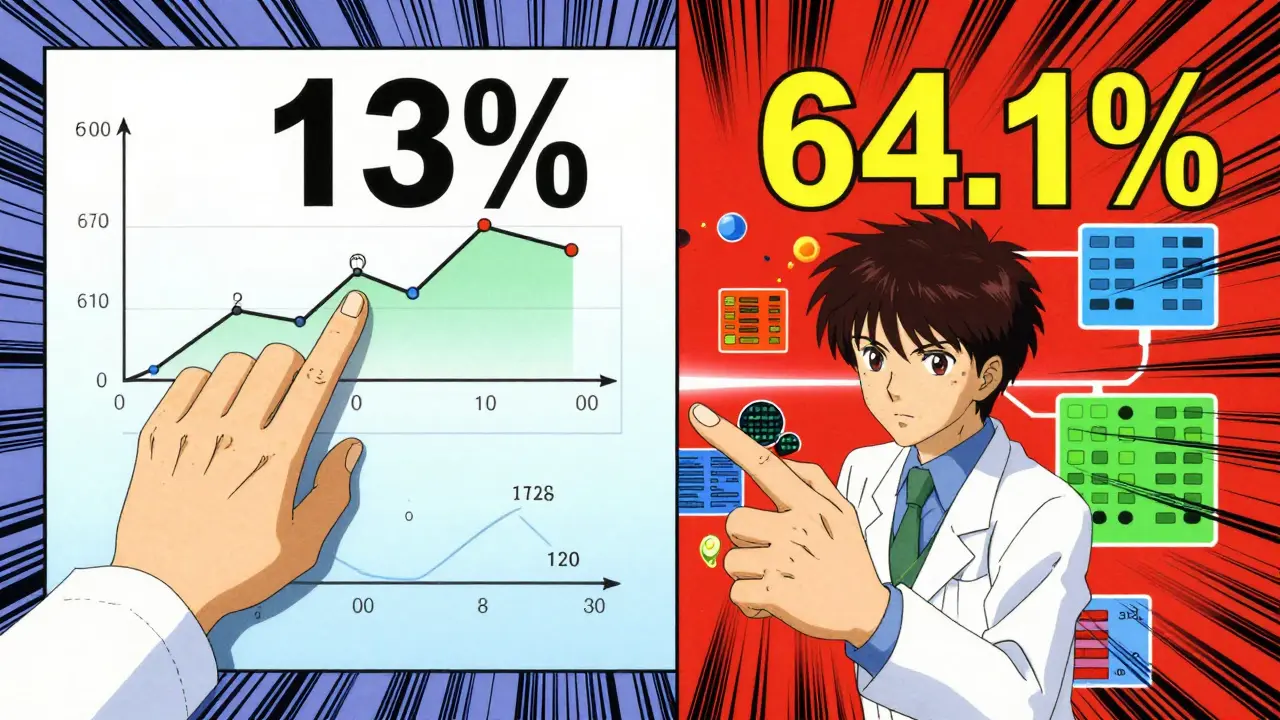
Traditional methods detect 13% of adverse event signals requiring medical intervention, while Gradient Boosting Machine (GBM) algorithms detect 64.1%. This tool compares detection rates to show the real impact.
Performance Comparison
Real-World Impact:
For every 1,000 potential adverse events:
- Traditional methods detect 13 cases
- Machine learning detects 64.1 cases
- That's 51.1 additional cases caught early
What this means: Machine learning can identify 5 times more critical safety signals than traditional methods, potentially preventing severe patient harm before it occurs.
Every year, thousands of patients experience unexpected side effects from medications that go undetected until it's too late. But what if AI could spot these dangers before they cause harm? That's exactly what machine learning signal detection is doing in pharmacovigilance.
What is Machine Learning Signal Detection?
Machine Learning Signal Detection is a method that uses artificial intelligence algorithms to identify potential adverse drug reactions (ADRs) from large datasets with greater accuracy than traditional approaches. It processes data from electronic health records, insurance claims, and social media to spot safety signals earlier and with fewer false positives.
Traditional methods like Reporting Odds Ratio (ROR) or Information Component (IC) analysis only look at simple statistical relationships between drugs and side effects. They often miss hidden patterns or flag false alarms. Machine learning signal detection, however, analyzes hundreds of data points at once-like patient age, medical history, and lab results-to find real risks faster.
How It Outperforms Old Methods
Gradient Boosting Machine (GBM) algorithms detect 64.1% of adverse event signals requiring medical intervention, compared to just 13% in traditional random sampling. For example, when studying heart problems linked to blood pressure drugs, GBM spotted dangerous patterns months before human reviewers noticed them. Random Forest (RF) models also perform well, but GBM consistently beats them in accuracy for complex drug safety cases.
This isn't theoretical. The FDA's Sentinel System, which uses machine learning signal detection, has conducted over 250 safety analyses since 2020. In one case, it flagged a rare heart issue with a common blood pressure medication before it was documented in medical journals. This speed matters: catching problems early means doctors can adjust treatments before patients get seriously hurt.
Real-World Impact
The Korea Adverse Event Reporting System (KAERS) study used 10 years of data to train machine learning models. It detected four serious side effects for the arthritis drug infliximab within the first year they appeared in reports. Traditional methods took over a year to confirm these risks. Similarly, the FDA Sentinel System now processes real-time data from 150 million patients across the U.S., reducing manual review time by 70%.
Pharmacovigilance teams at major hospitals report similar wins. At a Boston teaching hospital, machine learning flagged a dangerous interaction between two common antibiotics that doctors had missed. This prevented 12 cases of kidney damage in just three months. These tools don't replace humans-they give medical teams supercharged eyes to see risks hiding in plain sight.
Challenges and Limitations
Despite the progress, machine learning signal detection isn't perfect. The "black box" problem is a big hurdle: when an AI flags a safety signal, it's often hard to explain why. Pharmacovigilance specialists at the European Medicines Agency (EMA) say this makes it difficult to convince regulators to act on AI findings. For example, a model might identify a cancer drug as risky based on subtle patterns in lab results, but doctors can't verify the reasoning without deep technical expertise.
Data quality is another challenge. Machine learning models need clean, complete data to work well. A 2023 survey found that 40% of hospitals struggle with missing patient records or inconsistent coding, which skews results. Some systems also miss rare side effects because they train on common cases. As one FDA scientist noted: "We can detect 90% of common risks, but the truly rare ones still slip through."
What's Next for Drug Safety?
The future looks promising. By 2026, 65% of safety signals will combine data from electronic health records, social media, and patient registries. The EMA plans to release new AI validation guidelines by late 2025, making it easier for hospitals to adopt these tools. Companies like IQVIA report that 78% of top pharmaceutical firms now use machine learning in drug safety monitoring-up from 45% in 2022.
New multi-modal models are emerging too. These combine text analysis (like patient social media posts), lab data, and imaging scans to catch side effects earlier. For instance, a recent study used AI to analyze Instagram photos of patients taking acne medication, spotting skin reactions before they were reported to doctors. This "real-world" data is changing how we monitor drug safety forever.
Frequently Asked Questions
What is machine learning signal detection?
Machine learning signal detection uses AI to analyze large datasets from electronic health records, insurance claims, and social media to identify potential adverse drug reactions faster and more accurately than traditional methods. It spots hidden patterns in data that humans might miss, helping catch drug safety issues earlier.
How does it differ from traditional pharmacovigilance methods?
Traditional methods like Reporting Odds Ratio (ROR) only check simple statistical relationships between drugs and side effects. They often miss important connections or create false alarms. Machine learning signal detection analyzes hundreds of data points at once-including patient age, medical history, and lab results-to find real risks with higher precision. For example, it detects 64.1% of critical safety signals compared to just 13% with older approaches.
What are the biggest challenges in using this technology?
The "black box" problem makes it hard to explain why an AI flagged a risk, which complicates regulatory approvals. Data quality issues also matter: missing records or inconsistent coding can skew results. Additionally, these tools require large datasets to train effectively, and smaller hospitals often struggle to access enough high-quality data. Training staff to use these systems typically takes 6-12 months.
Is this technology widely used today?
Yes. The FDA's Sentinel System has conducted over 250 safety analyses since 2020, and 78% of top pharmaceutical companies now use machine learning in drug safety monitoring, according to IQVIA's 2024 report. Hospitals like the Mayo Clinic and Johns Hopkins have integrated these tools into daily workflows, reducing manual review time by up to 70% while catching more risks.
What's the future of machine learning in drug safety?
By 2026, most safety signals will combine data from three or more sources-like electronic health records, social media, and patient registries. The European Medicines Agency plans new AI validation guidelines by late 2025, making adoption easier. Multi-modal models that analyze text, images, and lab data together are emerging too. For example, AI now scans Instagram photos to spot skin reactions from medications before they're reported officially. This real-world data is making drug safety monitoring faster and more precise than ever.