Walk into any pharmacy in Bristol, and you’ll see rows of vitamins and supplements right next to painkillers and cold medicines. They look alike. They sit on the same shelf. You might even grab one without thinking twice-after all, it’s just a pill, right? But here’s the truth: OTC vitamins and supplements are not regulated like OTC medications. And that gap could be risking your health.
The Label That Tricks You
You’ve seen it before: a small rectangular panel on the back of a bottle. It’s called the Supplement Facts label. It looks almost identical to the Drug Facts label on your ibuprofen or allergy pill. Same layout. Same fonts. Same spacing. But that’s where the similarity ends. The Drug Facts label is the law. Since 1999, the FDA has required every OTC medication to list nine specific things: active ingredients with exact milligram amounts, what it’s for, when not to use it, possible side effects, drug interactions, inactive ingredients, dosage instructions, storage info, and expiration dates. Every single one. No exceptions. Supplements? Not even close. The Supplement Facts label only needs to list the serving size and the amount of each ingredient. That’s it. No warnings about interactions with your blood pressure meds. No clear guidance on how long you should take it. No pregnancy warnings-even if the product contains vitamin A at levels known to cause birth defects. A 2021 study in JAMA Internal Medicine found that only 17% of supplement labels mention potential drug interactions. Meanwhile, 100% of OTC medication labels do. That’s not a mistake. That’s the system.What’s Missing? The Hidden Dangers
Let’s talk about vitamin A. Prescription acne drugs like isotretinoin come with thick warning labels, mandatory pregnancy tests, and contraception requirements. Why? Because too much retinol can cause severe birth defects. Now look at your multivitamin. It might have 10,000 IU of vitamin A-same amount. But the label? It might say “Vitamin A (as retinol)” and nothing else. No bold warning. No pregnancy alert. Just buried in fine print, if it’s there at all. And here’s the kicker: vitamin A on supplement labels is often listed in International Units (IU). But IU doesn’t tell you if it’s retinol (dangerous in pregnancy) or beta-carotene (safe). You can’t tell the difference unless you dig into the ingredient list-and most people don’t. Then there’s sodium. If you have high blood pressure, you’re told to watch your salt intake. OTC painkillers like aspirin or naproxen must list sodium content per dose. Supplements? Not a single one has to. A 2022 analysis by the American Medical Association found that some popular energy supplements contain more sodium than a bag of chips-without any warning. And what about blends? You’ve seen them: “Proprietary Blend: 500mg.” That means the company doesn’t have to say how much of each ingredient is in there. One brand’s “Immune Support Blend” might have 10mg of zinc. Another’s might have 50mg. You won’t know. And if you’re already taking a prescription immune modulator? You could be overdoing it-and not even realize it.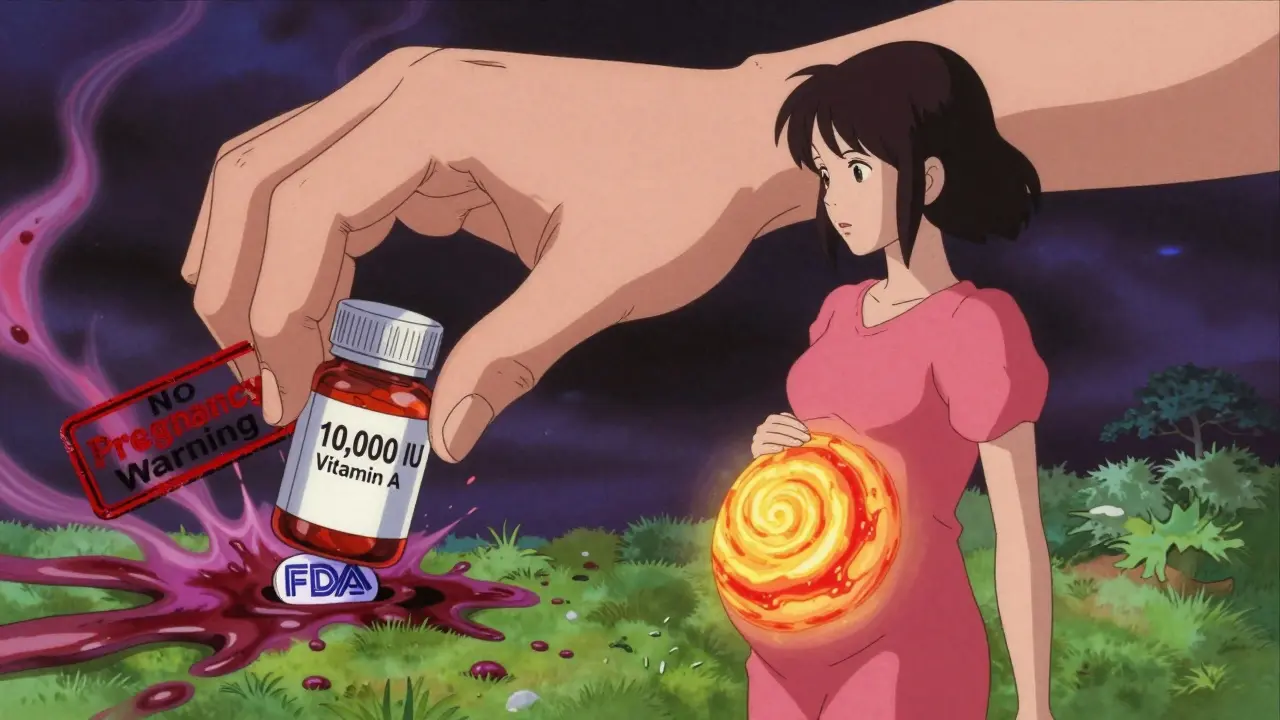
Who’s Really Checking These Products?
The FDA doesn’t approve supplements before they hit the shelf. They don’t test them. They don’t require safety studies. The law says supplements are food. That means they’re assumed safe until proven dangerous. Between 2008 and 2020, the FDA found 776 dietary supplements containing hidden pharmaceutical drugs-like erectile dysfunction meds, weight-loss stimulants, or steroids. These weren’t mislabeled. They were outright frauds. And the labels? Still said “natural ingredients.” The FDA can only act after someone gets hurt. And even then, it takes them an average of 427 days to remove a dangerous product. For OTC medications? 45 days. Dr. Pieter Cohen from Harvard Medical School put it bluntly: “The current labeling creates a false sense of security.” People assume that because it’s sold in a pharmacy, it’s been checked. It hasn’t.Why Does This Keep Happening?
The supplement industry made $54.2 billion in the U.S. in 2022. That’s more than the entire UK’s annual spending on prescription drugs. With that kind of money comes serious lobbying power. In 2022, supplement companies spent $8.2 million on federal lobbying to block stricter labeling rules. They fought off bills that would’ve required them to list interactions, pregnancy warnings, or sodium content. The Dietary Supplement Health and Education Act (DSHEA) of 1994 is still the law-and it’s outdated. Even the FDA admits it. In June 2023, they released a draft proposal asking supplement makers to list vitamin A in mcg RAE (a more accurate unit) and add clear pregnancy warnings. But it’s just a suggestion. No enforcement. No deadlines. Meanwhile, the market keeps growing. Sales are up 8.3% every year since 2019. And more people are buying supplements than ever. But they’re still trusting labels that don’t tell the full story.
What You Can Do
You can’t rely on the label. So here’s how to protect yourself:- Check the active ingredient amounts. If it says “500mg of vitamin D,” that’s fine. If it says “Proprietary Blend: 500mg,” walk away. You don’t know what you’re getting.
- Look up the product on Examine.com or ConsumerLab.com. These sites test supplements for accuracy and contamination. They’re free, independent, and trustworthy.
- Ask your pharmacist. Walgreens pharmacists logged over 14,000 questions in early 2023 about supplement safety. They’ve seen it all. Don’t be shy.
- Never assume safety because it’s “natural.” Poison ivy is natural. Snake venom is natural. So is high-dose vitamin A.
- Read the disclaimer. If it says “This product is not intended to diagnose, treat, cure, or prevent any disease,” that’s the FDA’s way of saying: “We didn’t check this.”
The Bottom Line
OTC vitamins and supplements aren’t dangerous because they’re bad. They’re dangerous because we think they’re regulated like medicine. They’re not. And that misunderstanding is costing people their health. You wouldn’t take a pill without reading the label. But if you’re taking a supplement, you’re taking it without knowing half the story. The label doesn’t warn you about interactions. It doesn’t tell you if it’s safe during pregnancy. It doesn’t even tell you how much of each ingredient is really in there. The FDA isn’t coming to fix this anytime soon. The industry won’t fix it on its own. So you have to. Be the one who reads beyond the label. Be the one who asks the questions. Your body doesn’t care if it’s called a “vitamin” or a “drug.” It only cares about what’s inside.Do OTC vitamins have to list drug interactions like painkillers do?
No. Unlike OTC medications, which are legally required to list all known drug interactions, supplement labels only have to list ingredients. Only about 17% of supplement labels mention interactions at all, according to a 2021 JAMA study. That means you could be mixing a vitamin with your blood thinner or antidepressant without knowing the risk.
Why don’t supplement labels show sodium content?
OTC medications must list sodium per serving because it affects blood pressure and heart health. Supplements don’t have that requirement-even if they contain salt-based ingredients like sodium ascorbate or sodium bicarbonate. A 2022 analysis found some energy supplements had more sodium than a bag of chips, with no warning on the label.
Is it safe to take high-dose vitamin A supplements during pregnancy?
No. Vitamin A in the form of retinol (not beta-carotene) is linked to birth defects at doses over 10,000 IU per day. The American College of Obstetricians and Gynecologists found that 40% of prenatal vitamins exceed this limit. But only 22% of those products have clear pregnancy warnings. Always check the form of vitamin A-and talk to your doctor before taking any supplement during pregnancy.
What does “proprietary blend” mean on a supplement label?
It means the manufacturer doesn’t have to tell you how much of each ingredient is in the blend. For example, a “Weight Loss Blend” might say 500mg total-but that could be 490mg of filler and 10mg of actual active ingredient. This is common in protein powders and fat burners. If a product uses blends, it’s harder to know if you’re getting enough-or too much-of any one component.
Can the FDA remove unsafe supplements from the market?
Yes-but only after harm is reported. Unlike OTC drugs, which must be proven safe before sale, supplements are assumed safe until proven dangerous. The FDA must prove a product is adulterated or misbranded to remove it. On average, it takes 427 days to act on a dangerous supplement. By then, thousands may have already taken it.
Are there any trustworthy labels to look for on supplements?
Yes. Look for third-party certifications like NSF Certified for Sport, USP Verified, or ConsumerLab.com Approved. These mean the product was independently tested for ingredients, contaminants, and accurate labeling. As of 2023, only about 2,147 products had NSF certification-less than 1% of the market. Don’t assume a label is trustworthy unless it’s certified by a third party.
Should I trust supplements sold on Amazon or Instagram?
Be extremely cautious. A 2023 review of 5,000 Amazon reviews for top-selling multivitamins found that 32% of 1-star reviews cited confusion over dosage or lack of warnings. Many products sold online contain undeclared pharmaceuticals, heavy metals, or incorrect dosages. Stick to reputable brands with third-party testing and avoid products with exaggerated claims like “miracle cure” or “doctor-recommended” without proof.